Introduction
Platelets are anuclear cells released from megakaryocytes, the rare myeloid cells that undergo multiple rounds of endomitosis before releasing platelets. Platelets, known as the mediator of thrombosis, are emerging as cellular mediators of type Ⅱ diabetes, atherosclerosis, cancer cell metastasis, and immune responses [1,2]. Despite the lack of a nucleus, they express a large number of transcription factors including peroxisome proliferator-activated receptor (PPAR). PPAR needs ligand-binding to be activated [3].
PPAR, a ligand-activated transcription factor, consists of 3 isoforms: α, β/δ, γ, and requires heterodimerization with retinoid X receptor to modulate transcription of target genes [4]. PPARγ is involved in glucose metabolism, inflammation, and differentiation and functions of adipose tissue [5-7]. Recent studies have reported that the PPARγ inhibits platelet activation and aggregation [8-11]. Also, studies have reported the PPARγ and ligand of PPARγ as the factor influences the hematopoietic system. They could affect the roles of erythroid, myeloid, monocytic, and lymphocytic cell function during an immune response, and modulate proliferation and maturation of erythroid progenitor cells [12-14]. The endogenous 15-deoxy-Δ [12,14] prostaglandin J2 (15d-PGJ2) is a ligand of PPARγ. Biologically, 15d-PGJ2 is the most active metabolite of prostaglandin D2 [15].
Fucoidans, fucose-containing sulfated polysaccharides, are constituents of brown seaweed and some marine invertebrates. Extensive studies have reported their various biological effects including immunostimulation, anti-tumor, antiviral, antithrombotic and anticoagulant activities. By far the anticoagulant and antithrombotic actions of fucoidans are the most widely recognized bioactivities, but the basis for these activities is not completely understood [16-19].
We are interested in the influences of 15d-PGJ2 and fucoidans to the megakaryocytic differentiation and the platelet production, as well as the influences of 15d-PGJ2 and fucoidans to the thrombosis and coagulation system. Due to their antithrombotic and anticoagulant activities, they have the probability of the application as the novel therapeutic agents to cardiovascular disease. Also, if they have the abilities inducing the production of platelet, they could be the stable solution of platelet supply to the thrombocytopenic patients.
In this study, we evaluated the effects of 15d-PGJ2 and fucoidans on megakaryocytes and platelets with diverse methods from protein to the molecular level. This study aims to determine 1) whether the 15d-PGJ2 and fucoidans encourage the megakaryocytes to produce platelets actively; 2) whether the 15d-PGJ2 and fucoidans possess the anticoagulant and antithrombotic actions, and 3) whether specific factors, such as prostaglandins or genes, explain the mechanism of how the 15d-PGJ2 and fucoidans influence differentiation and function of the platelets. To search these factors, we also included PPARα, which activates fatty acid catabolism and stimulates gluconeogenesis, attenuates inflammatory responses, and participates in the amino acid metabolism, and COX-2, which is responsible for the formation of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid.
Methods
Platelet isolation
Platelets were isolated from discarded plateletpheresis products by centrifugation at 1,800 g for 8 minutes at room temperature. CBC with platelet count was done using Sysmex XE-2100 automated hematology system (Sysmex Corporation, Japan). On average, we obtained 1.3×106/μL platelets for the experiment, and the platelet purity was greater than 99%.
Cell culture
We purchased the human megakaryocytic DAMI cell lines and human megakaryoblastic Meg-01 cell lines from Sigma company (Balcatta, WA). DAMI and Meg-01 cells were cultured in RPMI-1640 tissue culture medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS; Invitrogen).
Platelet, DAMI and Meg-01 cells treatment
We treated platelets, DAMI, and Meg-01 cells with 10 μM 15d-PGJ2 (PPARγ ligand), 20 μM GW9662 (PPARγ antagonist), and 50 μg/mL fucoidan for 24 hours.
We also treated platelets with 50 μg/mL fucoidan to examine the expression of PPARα and COX-2 in the presence or absence of 0.25 μM GW6471 (PPARα antagonist) and 0.04 μM celecoxib (COX-2 inhibitor) for 24 hours.
Microscopic observation
Cytospin smears of DAMI and Meg-01 cells were stained with Wright-Giemsa and examined after each experiment. To investigate the megakaryocytic differentiation and platelet formation effect of 15d-PGJ2, GW9662, and fucoidan on the DAMI cells, we observed the morphology of those cells with an optical microscope.
Flow cytometry
Platelets, DAMI, and Meg-01 cells were identified using CD61 (glycoprotein Ⅲa) antibody. They were analyzed by staining with CD41 (glycoprotein Ⅱb/Ⅲa), CD42b (glycoprotein Ⅰb), CD62p (P-selectin), CD63 (platelet activation marker) and PAC-1 antibodies and using a Coulter Navios flow cytometer (Beckman Coulter, USA). Data acquisition and analysis were performed using Kaluza analysis software (Beckman Coulter, USA).
Western blotting
We extracted proteins by a standard technique [8], and diluted aliquots (30 μg) with 4× Loading buffer (Invitrogen, USA), heated at 95℃ for 5 minutes and fractionated by 8% polyacrylamide gel electrophoresis. To compare the expression of PPARγ, PPARα and COX-2 in human megakaryocytes and platelets, the 1:2,000 diluted primary goat antibody and rabbit anti-goat antibody were used, respectively, for 2 hours at room temperature. Membranes were visualized with chemiluminescence.
Thrombus formation analysis
Total thrombogenicity of platelets treated with fucoidan was measured by T-TAS (Total Thrombus-formation Analysis System; Fujimori Kogyo, Japan). The system is developed for the quantitative assessment of the platelet thrombus formation (PTF) process. There are two types of the microchip, “PL-chip” and “AR-chip” for evaluating “platelet-specific” and “comprehensive” thrombus formation, respectively. When blood flows through the chip, the platelet aggregates gradually increase the flow pressure. In PTF-assay, the parameter AUC (area under the flow pressure curve) represents total thrombogenicity.
Microarray analysis
The effect of fucoidan on DAMI cells was evaluated with microarray analysis. We used 100 ng of total RNA for generation of biotin-labeled cRNA using Affymetrix Two-Cycle cDNA Synthesis Kit (Affymetrix, Santa Clara, CA). The labeled cRNA was cleaned, quantified and after fragmentation, hybridized to Affymetrix HG-U133 Plus2.0 GeneChips. The chips were stained with streptavidin-phycoerythrin and biotinylated antibody and washed at an Affymetrix Fluidics station 450. The GeneChips were scanned and data extracted using GeneChip scanner 3000 (Affymetrix, Santa Clara, CA), and the raw data file formats were generated using GeneChip operating software (GCOS).
Results
The effect of 15d-PGJ2 (PPARγ ligand) on megakaryocytes
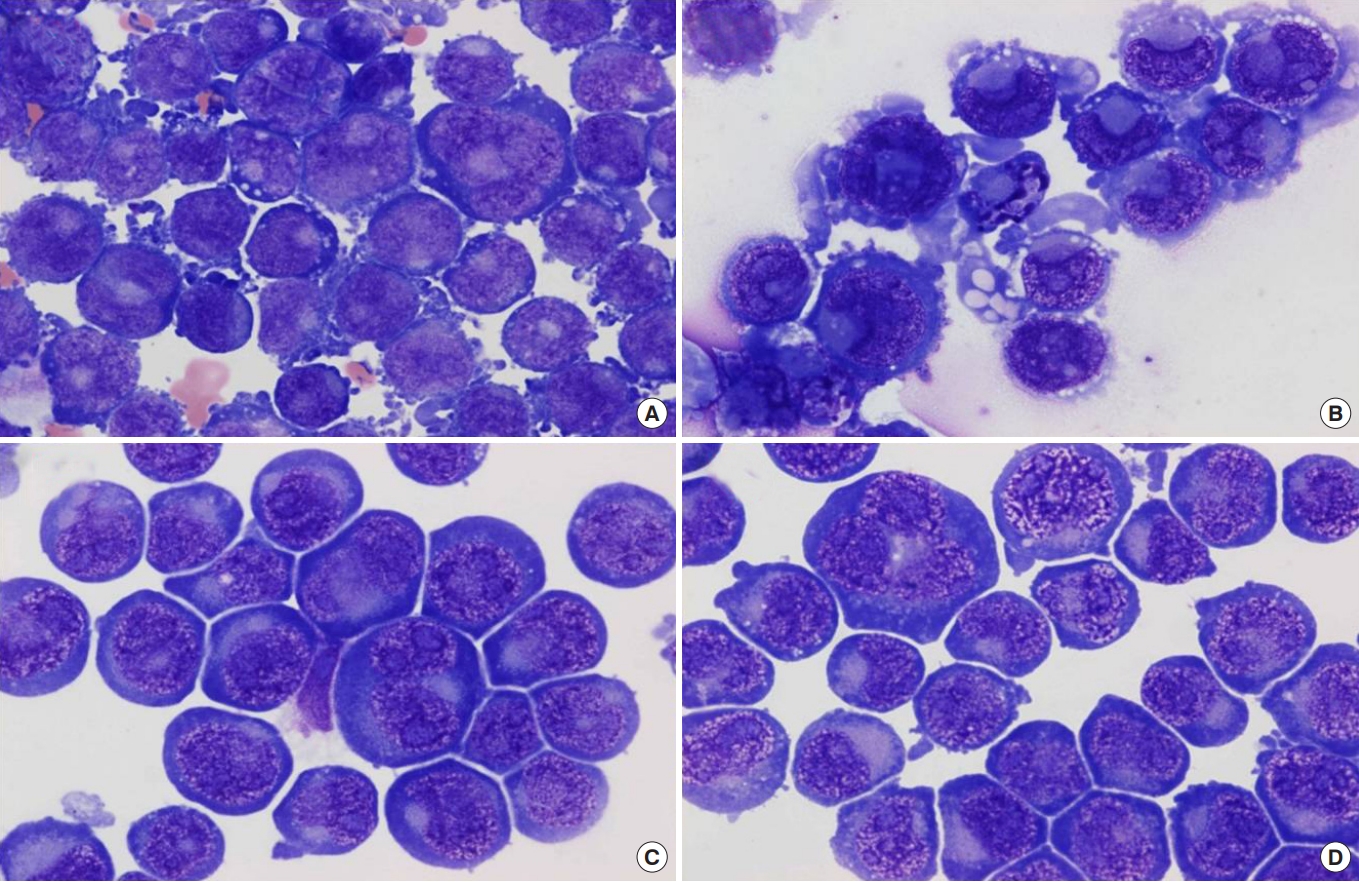
We have demonstrated that 15d-PGJ2 induced morphological differentiation in DAMI cells, reflective of megakaryocyte maturation. 15d-PGJ2-treated DAMI cells showed the smaller size of nuclei and a more abundant amount of cytoplasm with less basophilic color, suggesting differentiation evidence (Fig. 1).
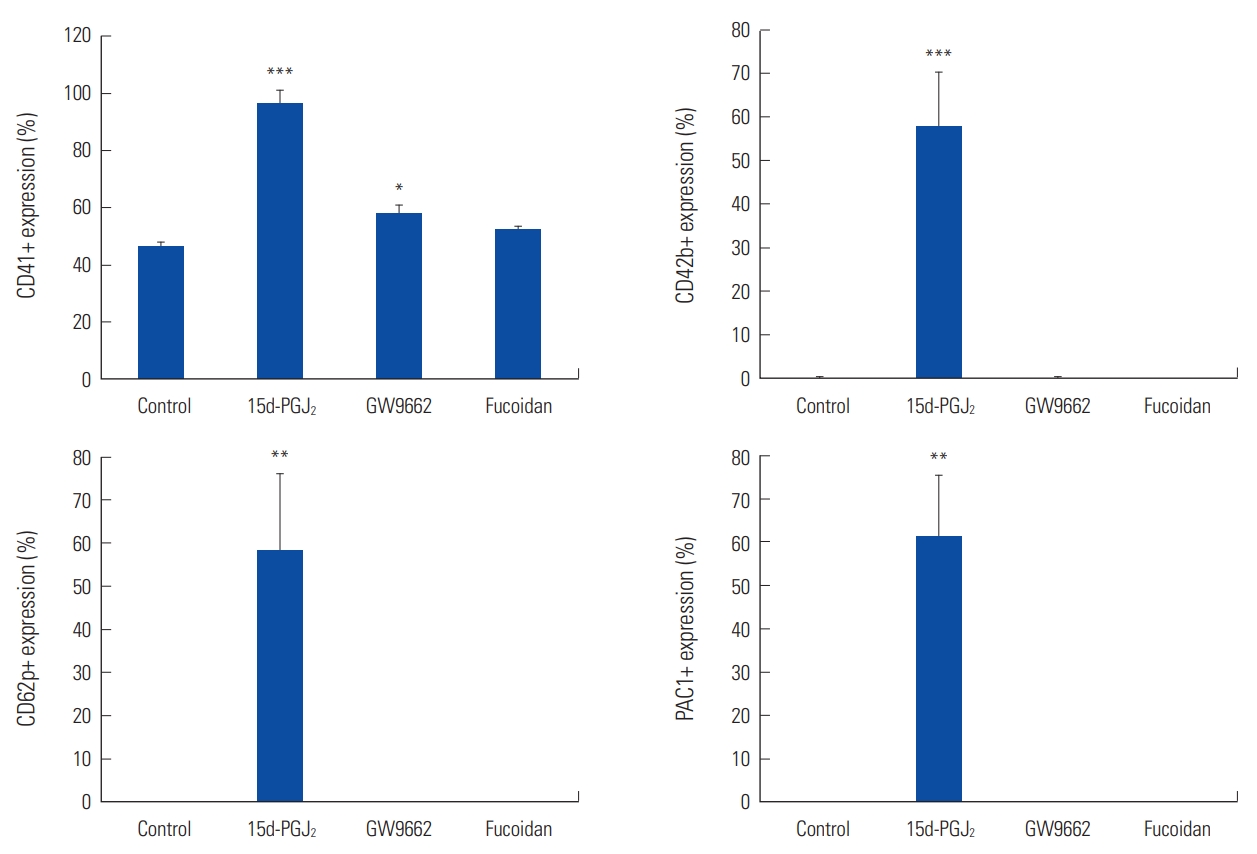
Increased levels of CD41, CD42b, CD62p, and PAC-1 by 15d-PGJ2 in DAMI cells also indicate differentiation of megakaryocytes into mature cells. 15d-PGJ2 increased the levels of CD41 (P=0.0004), CD42b (P=0.0008), CD62p (P=0.004) and PAC-1 (P=0.001) significantly in DAMI cells, respectively (Fig. 2).
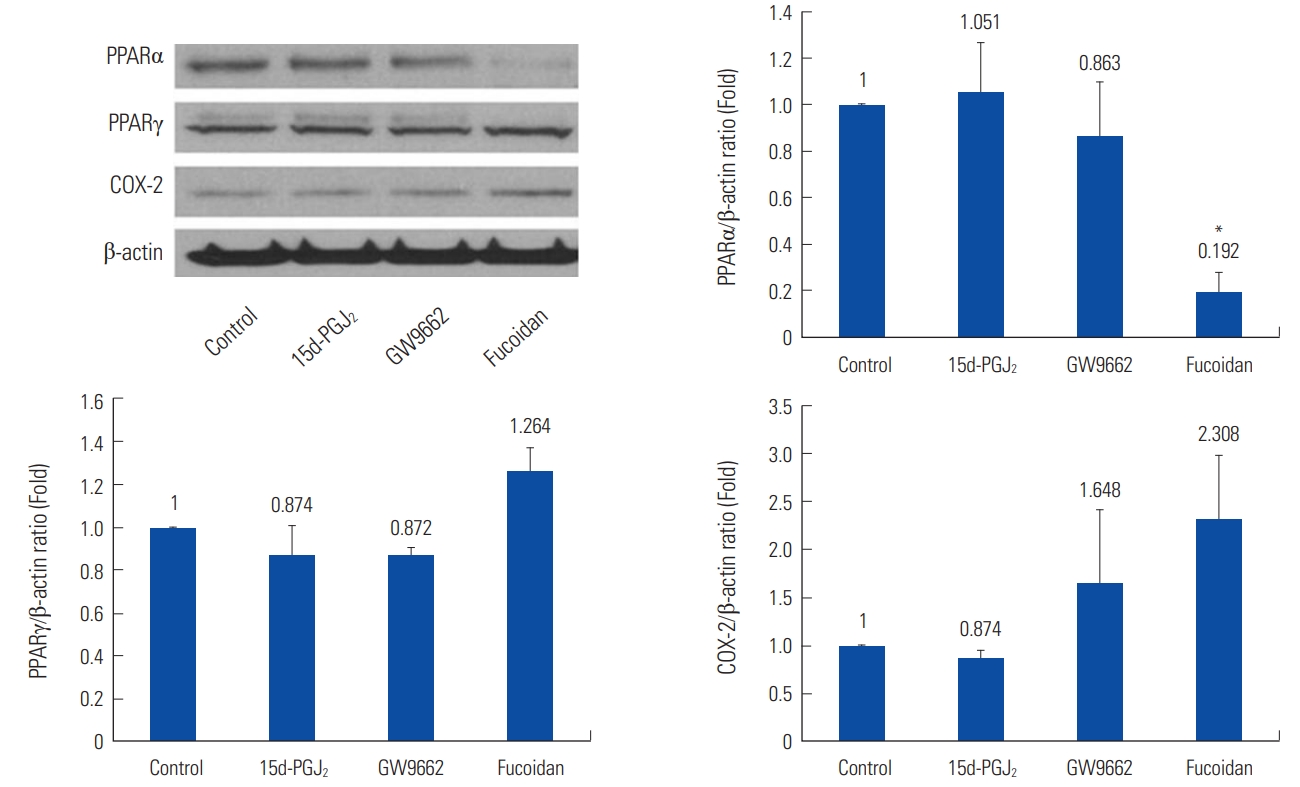
15d-PGJ2 had no significant effects on the expression of PPARγ in megakaryocytes. In DAMI cells, 15d-PGJ2 decreased COX-2 expression (0.559 fold, P<0.01) than control (Fig. 3).
The effect of GW9662 (PPARγ antagonist) on megakaryocytes
Treatment with GW9662, the irreversible PPARγ antagonist, induced a few specific changes. GW9662-treated DAMI cells showed no specific morphological changes, although gave lower N (nucleus)/C (cytoplasm) ratio as compared to the control (Fig. 1).
Except for significantly increased level of CD41 (P=0.039), GW-9662 had no significant effect on the level of glycoproteins, especially on CD42b, CD62p, and PAC-1 in DAMI cells (Fig. 2).
In the Western blotting to compare the expression of PPARγ, PPARα, and COX-2, GW9662 had no significant effects (Fig. 3).
The effect of fucoidan on megakaryocytes
We also evaluated the effect of fucoidan on the platelet differentiation. We expected fucoidan has an effect on the production of platelets, but we could not observe any morphological changes as 15d-PGJ2 (Fig. 1).
Additionally, fucoidan had no significant effect on CD41, CD42b, CD61, CD62p, CD63, and PAC-1 in DAMI cells (Fig. 2).
Fucoidan had no significant effects on megakaryocytes in the Western blotting to compare the expression of PPARγ, PPARα, and COX-2 (Fig. 3).
The effect of fucoidan on DAMI cells was evaluated with microarray analysis. As results, 17 transcript clusters were differentially expressed (P<0.05) at a >2.0 fold change. Among the clusters, five clusters were mapped to a valid gene, the early growth response 1 (EGR1), Fc receptor, IgG, high affinity 1 (FCGR1), YME1 like 1 ATPase (YME1L1), stanniocalcin 1 (STC1) and cytochrome P450 family 11 subfamily B member 2 (CYP11B2).
The effect of 15d-PGJ2 and GW9662 on mature platelets
15d-PGJ2 and GW9662 showed no significant effects in mature platelets.
The effect of fucoidan on mature platelets
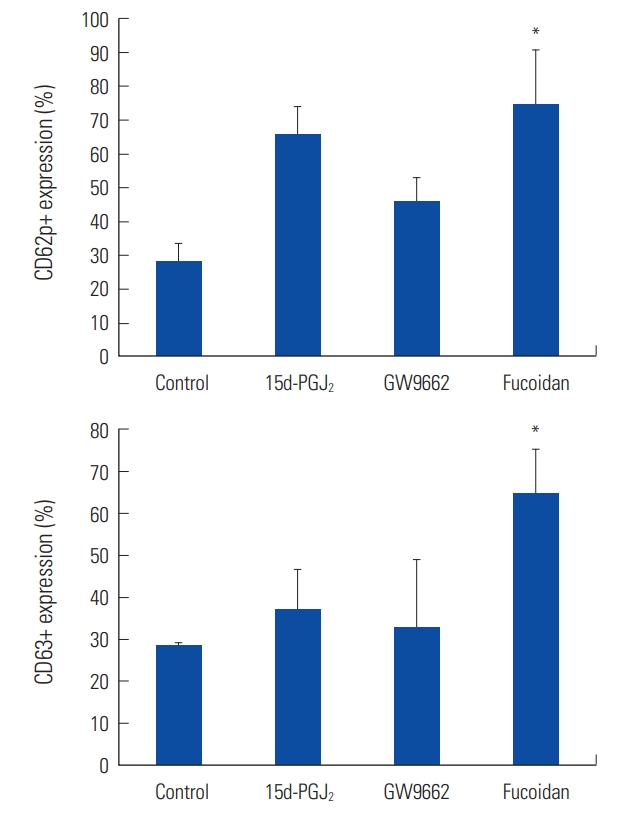
In flow cytometry analysis, fucoidan elevated the expression of CD62p (P=0.033) and CD63 (P=0.036) significantly (Fig. 4).
In mature platelets, fucoidan decreased PPARα expression (0.192 fold, P= 0.014), and increased COX-2 expression (2.308 fold, P=0.218) than control (Fig. 5).
Fucoidan increased COX-2 expression in combination with GW6471 (2.115 fold, P=0.109), and combination with celecoxib (2.97 fold, P=0.099) than control.
PTF-assay with T-TAS was performed. In the study with PL-chip, AUC of GW6471 (43.5) was wider than control (28.35) (P=0.970), AUC of fucoidan (8.55) was narrower than control (P=0.916), and AUC of celecoxib (28.85) was similar with control (P=1.000). In the study with AR-chip, AUC of GW6471 (44.7) was wider than control (38.1) (P= 0.998), AUC of celecoxib (28.75) and fucoidan (27.4) was narrower than control (P=0.992, P=0.985, respectively).
Discussion
Megakaryocytes are the biggest cell of the bone marrow, and platelets are derived from the cytoplasm of megakaryocytes. Platelets are metabolically active cellular mediators of various vascular biology containing copious amounts of bioactive materials including thromboxanes, prostaglandins, chemokines, and cytokines. Besides their crucial function of initiating thrombosis and maintaining hemostasis, platelets are also revealed as key mediators of inflammation and immune cell activation [1,2].
While the clear importance of the platelets in various conditions and diseases has been emerging, diverse proteins to be expressed or released from platelets have been identified through proteomics. Although mature platelets are anucleated, they were discovered to express a large number of transcription factors including PPAR which is involved in the regulation of metabolism and inflammation and regulated by activation or treatment with PPAR ligands [3]. PPARγ ligands have been suggested to influence platelet production and function.
There are researches aimed to reveal the functions of the PPAR and ligand of PPAR influencing the hematopoietic system. The interesting thing is that the reports mentioned it is unclear if the PPARγ itself modulates the differentiation of megakaryocytes, whereas the PPARγ ligand enhances platelet production from megakaryocytes [12,14]. In our study, 15d-PGJ2 induced morphological differentiation in DAMI and Meg-01 cells, reflective of megakaryocyte maturation. Increased levels of CD41, CD42b, CD62p, and PAC-1 by 15d-PGJ2 in DAMI and Meg-01 cells also indicate differentiation of megakaryocytes into mature cells and platelet release. These results suggest 15d-PGJ2 could lead to enhanced platelet production. However, we couldn’t conclude it is dependent on the PPARγ because of the effects of GW9662, the PPARγ antagonist, on megakaryocytes. Although it induced a few changes on the morphology and flow cytometry analysis, the lower N (nucleus)/C (cytoplasm) ratio of megakaryocytes and significantly increased the level of CD41 indicate GW9662 could influence the differentiation of megakaryocytes slightly. So, the effects of 15d-PGJ2 on megakaryocytes are clear, but the probability of intermediation of PPARγ is somewhat lower.
15d-PGJ2 significantly decreased COX-2 expression of megakaryocytes. A study suggested 15d-PGJ2 use both receptor-dependent and -independent mechanisms to influence COX2 gene expression [20]. In the study, it was observed that COX2 expression was significantly abrogated by 15d-PGJ2. But they made an experiment on the influence of 15d-PGJ2 in Human WISH, the cells originated from amniotic sac, and amnion cells, further studies using megakaryocytes would be helpful to understand the precise mechanisms.
In our study, fucoidan had no significant effect on megakaryocytes in morphology, flow cytometry analysis, and Western blotting. But in the microarray analysis, the five genes were significantly upregulated in fucoidan-treated DAMI cells. The protein encoded by EGR1 is a nuclear protein and functions as a transcriptional regulator. FCGR, the receptors for the Fc region of IgG, is expressed by leukocytes and platelets. YME1L1 encodes the human ortholog of yeast mitochondrial AAA metalloprotease Yme1p, which has been proposed that the gene plays a role in mitochondrial protein metabolism. STC1 is a homodimeric glycoprotein that may play a role in the regulation of calcium and phosphate transport and cell metabolism. STC1 gene expression has altered in tumors. CYP11B2 encodes a member of the cytochrome P450 superfamily of enzymes, which synthesize aldosterone.
EGR1 has been reported to contribute to suppression of PPARγ expression [21]. To know the more accurate mechanism related to the PPARγ, the microarray analysis using ago- and antagonists of PPARγ along with fucoidan could be helpful. EGR1 has also been reported to regulate the other gene in megakaryocytes [22]. So, EGR1 could influence megakaryocytes with other substances as well as PPARγ.
It has been reported that the expression of STC1 was strongly upregulated during megakaryocytic differentiation and accumulation of STC1 protein in normal megakaryocytes and platelet was observed [23].
CYP11B2 has been reported to be suppressed by PPARγ [24]. We suggest there is a negative correlation between fucoidan and PPARγ, but as we said earlier, the effect of PPARγ on megakaryocytes is not explained obviously, this correlation should be further studied. Functions of FCGR1 and YME1L1 in platelets are studied well, but their roles in megakaryocytes are not fully understood.
We couldn’t have meaningful results with platelets. There are several studies reporting PPARγ ligand to have an inhibitory effect on platelet activation. And fucoidan has been reported possessing anticoagulant and antithrombotic actions. In our study, the ligand and antagonist of PPARγ showed no significant effects in mature platelets. Fucoidan significantly elevated the level of CD62p and CD63 and decreased PPARα expression. With or without GW6471 or celecoxib, fucoidan increased COX-2 expression than control. In PTF-assay with T-TAS, GW6471 appeared to enhance thrombogenicity, whereas fucoidan appeared to suppress thrombogenicity. We guess the platelets isolated from discarded plateletpheresis products could have some limitations such as partial activation of platelets influencing the results.
Thrombocytopenia is a critical problem causing significant morbidity and mortality, and platelet transfusion is the most commonly used therapy but has limitations of alloimmunization, availability, and expense. In this study, we found that 15d-PGJ2 and fucoidan influence the development of megakaryocytes through regulation of PPARγ and genes including EGR1 and STC1. This finding suggests the possibility of development of more safe and convenient method using the small molecules to treat thrombocytopenic patients.
The PPARγ ligand could enhance megakaryocytic maturation independent on PPARγ, so studies for another mechanism of PPARγ ligand influencing megakaryocytes are needed. And the relationship between megakaryocytic differentiation and decrease of COX-2 expression by PPARγ ligand should be investigated further. Also, the hidden abilities of genes upregulated by fucoidan should be studied as well. In summary, these findings support PPAR ligand, and brown seaweed based compound plays a role in megakaryocytic differentiation even in genomic level.