AbstractPurposeThe present study sought to investigate the association between diabetes mellitus (DM) and platelet reactivity using three different platelet function tests in a Korean patient population.
MethodsBlood sampling for platelet function analyses was performed one-day after percutaneous coronary intervention (PCI) between 8 to 48 hours after the last administration of standard drugs including clopidogrel and aspirin. The platelet function assessments included VerifyNow (VN), multiple electrode aggregometry (MEA) and light transmittance aggregometry (LTA).
ResultsDM patients had significantly higher mean platelet reactivity units (PRU) compared to non-DM patients by VN in the overall data (DM vs. non-DM: 199.90± 106.00 vs. 185.99± 100.99, P= 0.019), but there were no significant differences in platelet reactivity by VN in the propensity score matching (PSM) data. No significant differences were determined by LTA or MEA assays between DM and non-DM patients in the overall and PSM data. High platelet reactivity (HPR) was more frequently observed in DM versus non-DM patients by VN (Overall: DM vs. non-DM: 34.7% vs. 24.9%, P= 0.001, PSM: DM vs. non-DM: 33.3% vs. 26.8%, P= 0.045), and no significant differences were detected in the incidence of HPR by LTA or MEA assays between the two groups in the overall and PSM data.
IntroductionDual antiplatelet therapy with aspirin and clopidogrel is the standard of care for patients undergoing PCI with stents, including DM patients [1,2]. DM is associated with a high risk of recurrent cardiovascular events [3]. Compared with non-DM patients, DM patients have an increased tendency to activate and aggregate platelets despite antiplatelet therapy [4-8]. In diabetes patients, insulin resistance and hyperglycemia are associated with low-grade inflammation, as well as chronic enhancement of oxidative stress, triggering endothelial dysfunction and promoting atherogenesis [9,10]. In general, oxidative stress and reduced antioxidant activity induced by hyperglycemia significantly augment in diabetic patients, subsequently leading to platelet activation and hyperreactivity [11]. Studies have consistently shown that DM patients have impaired clopidogrel-induced antiplatelet effects, leading to HPR [4-8].
HPR during treatment with clopidogrel has been consistently shown to be a strong risk factor for recurrent ischemic events after PCI [12,13]. HPR in diabetic patients is due to increased platelet function, including an increased response to stimulation by platelet aggregation agonists, adhesion to thrombogenic surfaces and platelet aggregation [14]. The present study sought to investigate the association between diabetes and platelet reactivity using three different platelet function tests in a Korean patient population.
MethodsStudy design and populationThis was a single center observational study conducted at the cardiology department of Dong-A University Hospital (Busan, Korea). Written informed consent was obtained from all patients. We enrolled 1,079 PCI treated patients receiving maintenance dual antiplatelet treatment (DAPT) (75 mg/day clopidogrel and 100 mg aspirin) to reduce the variation during the loading phase.
Patients ≥ 18 years of age who had undergone drug-eluting stent (DES) implantation without exclusion criteria were eligible for this study.
The exclusion criteria were as follows: hemodynamic instability, malignancies, active bleeding or bleeding diathesis, contraindication to antiplatelet agents, concomitant use of warfarin or glycoprotein IIb/IIIa receptor blocker, platelet count <80,000/mm3 or hematocrit < 30%, an aspartate aminotransferase (AST) concentration or an alanine aminotransferase (ALT) concentration ≥ 3 times the upper normal limit, significant hepatic dysfunction, treatment with ticlopidine, prasugrel, ticagrelor, or dipyridamole, cardiac arrest or cerebrovascular injury with three months.
Blood samplingBlood sampling for platelet function analyses were performed one-day post PCI between 8 to 48 hours after the last administration of routine drugs including clopidogrel and aspirin in the hospital ward. Platelet aggregation was assessed using three methods including the VN P2Y12 assay (Accumetrics, San Diego, CA, USA), MEA (Dynabyte Medical, Munich, Germany) and LTA (Chrono-Log, Havertown, PA).
Platelet function testsThe platelet function tests were performed by experienced laboratory personnel in accordance with the manufacturer’s instructions.
VN assayThe VN P2Y12 assay is a point-of-care (POC), turbidimetric assay that measures platelet function and was used according to manufacturer’s instructions [15]. Within the cartridge of the VN P2Y12 assay is a channel that measures inhibition of the ADP P2Y12 receptor. This channel contains ADP as a platelet agonist and prostaglandin E1 (PGE1) as a suppressor of intracellular-free calcium levels to reduce the nonspecific contribution of ADP binding to P2Y12 receptors. The VN results are expressed in PRU.
MEA assayMEA is a semi-automated POC system, assessing platelet reactivity in whole blood [16], and was used according to the manufacturer’s instructions. The output values are expressed in arbitrary aggregation units (AU) [16]. MEA was performed with a Multiplate Analyzer (Dynabyte Medical, Munich, Germany). Specifically, the adhesion and aggregation of platelets on sensor surfaces enhances the electrical resistance between two sensor electrodes. We used an ADP test (6.4 µM ADP) to monitor the antiplatelet effects of DAPT, mostly targeting clopidogrel. In the test cuvette, whole blood (300 µL) was diluted (1:2 vol/vol) with 0.9% NaCl solution for 6.4 µM, and ADP was stirred in for 3 minutes at 37°C, before ADP in the absence of PGE1 was added, and the increase in electrical impedance was recorded continuously for 6 minutes and converted into arbitrary AU. Approximately 8 AU correspond to 1 Ohm. The means of the two independent determinations were expressed as the area under the curve of aggregation tracing (AUC) in AU· min. The manufacturer recommends the use of arbitrary units (U) to simplify the expression of results (1 U= 10 AU · min= 1 AUC).
LTA assayIn accordance with the LTA standard protocol [17], blood samples were drawn into a 3.2% sodium citrate-containing tube (Greiner Bio-One GmbH, Frickenhausen, Germany) and processed within 2 hours. Platelet-rich plasma was prepared by centrifugation at 120 g for 10 minutes. After the collection of platelet-rich plasma, platelet-poor plasma was obtained from the remaining specimen by recentrifugation at 1,200 g for 10 minutes. The platelet-rich plasma was then adjusted to a platelet count of 250,000 per ml by adding plateletpoor plasma as needed. Light transmission was calibrated by a cuvette with platelet-rich plasma which was normalized as 0% and a second cuvette containing platelet-poor plasma that was normalized as 100%. Platelet function was measured after the addition of 10 mL ADP, before the curves were recorded for 6 minutes. The results are expressed as maximum platelet aggregation (MPA) within 6 minutes.
Statistical analysisFor baseline characteristics, platelet function values and the percentage of HPR, continuous variables are expressed as mean±standard deviation, while categorical variables are presented as absolute numbers and frequencies. Continuous data were analyzed using student’s t-test and chi-square test for categorical variables. Uni-variate and multivariate logistic regression analyses were performed to identify the risk factors of HPR built to evaluate the effect of DM on antiplatelet responsiveness. Variables with P<0.2 in the uni-variate analysis were then entered into the multi-variate logistic regression analysis using backward selection elimination to provide an odds ratio (OR) and 95% confidence interval (CI). The propensity score for each patient was calculated using a multiple factor logistic regression model that included age (years), sex, BMI, hypertension, CKD, dyslipidemia, current smoking, previous MI, previous PCI, previous CABG, previous CVA, Non-AMI, AMI, CK-MB, hemoglobin, creatinine, platelet count, and HbA1c. With the propensity score estimated, 372 pairs of patients in the DM group and the non-DM group were matched using a 1:1 nearest neighbor matching algorithm. A forest plot was generated to identify the incidence of HPR in the study group by VN and by MEA. Correlations between measures derived from different platelet function assays were evaluated using the Pearson rank correlation coefficient. P values of <0.05 were considered statistically significant, and statistical analyses were performed using IBM/SPSSv23.0 (IBM/SPSS, Chicago, IL, USA).
ResultsBaseline characteristics of the study patientsFrom November 2008 to December 2016, we enrolled 1,079 PCI treated patients receiving maintenance DAPT (75 mg/day clopidogrel and 100 mg aspirin). Overall, there were 464 patients in the DM group and 615 patients in the non-DM group. After PSM, the two groups had the same number of patients (N=372). Table 1 summarizes the baseline characteristics of the study group.
The baseline results reveal a mean age of 66 years old, with most of the patients being male in the two groups. Diabetic patients had a higher prevalence of hypertension, CKD and a lower prevalence of current smoking than the non-DM patients. Patients with DM had a more frequent history of MI, PCI, and CVA. Compared to the non-DM group, there were higher values for creatinine and HbA1c, and lower values for hemoglobin than the DM group (P< 0.05). After PSM, the baseline results showed no differences between the two groups except for HbA1c, with diabetic patients having higher values than non-DM patients (DM vs. non-DM: 7.48±1.34 vs. 5.95±0.61, P= 0.000) (Table 1).
Platelet function tests and HPR in the study groupIn the platelet function tests, patients with DM had significantly higher mean PRU values compared to the non-DM patients as determined by VN using the overall data (DM vs. non-DM: 199.90±106.00 vs. 185.99±100.99, P= 0.019), but there were no significant differences in platelet reactivity between the two groups when determined by VN using PSM data (DM vs. non-DM: 197.01±105.03 vs. 184.97± 104.00, P=0.117). No significant differences were observed in platelet reactivity by LTA or MEA between the DM and non-DM patients in the overall and PSM data.
HPR was more frequently observed in DM patients versus non-DM by VN (Overall: DM vs. non-DM: 34.7% vs. 24.9%, P= 0.001, PSM: DM vs. non-DM: 33.3% vs. 26.8%, P= 0.045), and no significant difference existed in the incidence of HPR when determined by MEA or LTA between the two groups in the overall and PSM data (Table 2).
Logistic regression results from predictors of HPR by VNUnivariate analysis showed that gender (female), hypertension, DM, CKD and age were risk factors associated with HPR, whereas current smoking and hemoglobin were risk factors inversely related to HPR.
Multivariate logistic regression analysis showed that a lower hemoglobin value was a risk factor associated with HPR (OR, 0.776; 95% CI, 0.719 to 0.838; P< 0.001). DM was a significant predictor of HPR (OR, 1.432; 95% CI, 1.088 to 1.885; P= 0.010), and gender (female) (OR, 1.376; 95% CI, 1.015 to 1.867; P= 0.040) was significantly associated with the risk of HPR (Table 3).
Logistic regression results from predictors of HPR by MEAUnivariate analysis showed that a higher platelet count value was a risk factor associated with HPR (OR, 1.008; 95% CI, 1.005 to 1.012; P< 0.001).
Multivariate logistic regression analysis also showed that a higher platelet count value was a risk factor associated with HPR (OR, 1.008; 95% CI, 1.005 to 1.012; P< 0.001) (Table 4).
Logistic regression results from predictors of HPR by LTAUnivariate analysis showed that CKD and platelet count were risk factors associated with HPR, whereas hemoglobin was a risk factor inversely related to HPR.
Multivariate logistic regression analysis showed that CKD was a risk factor associated with HPR (OR, 1.738; 95% CI, 1.114 to 2.710; P= 0.015) and a higher platelet count value was a risk factor associated with HPR (OR, 1.003; 95% CI, 1.001 to 1.005; P= 0.004) (Table 5).
Comparison of HPR incidence in the study group by VN, MEA, and LTAUsing VN: For both overall and PSM patients, patients with DM had a higher incidence of HPR than non-DM patients (overall OR, 1.461; 95% CI, 1.097 to 1.945; P= 0.010, PSM OR, 1.360; 95% CI, 1.103 to 1.863; P= 0.046).
Using MEA: For both overall and PSM patients, no significant difference was observed in the incidence of HPR between the two groups (overall OR, 1.322; 95% CI, 0.759 to 2.301; P= 0.324, PSM OR, 1.677; 95% CI, 0.884 to 3.180; P= 0.114).
Using LTA: For both overall and PSM patients, there were no significant differences in the incidence of HPR between DM and non-DM patients (overall OR, 0.973; 95% CI, 0.721 to 1.314; P= 0.858, PSM OR, 0.914; 95% CI, 0.651 to 1.284; P= 0.603) (Fig. 1).
Comparison of platelet function testsThe correlation between MEA and VN measurements showed the lowest values (r= 0.345, P= 0.000) (Fig. 2A), while the correlation between LTA and VN measurements showed the highest values (r= 0.632, P= 0.000) (Fig. 2B). Meanwhile the correlation between LTA and MEA measurements showed values that were between the highest and lowest (r= 0.495, P= 0.000) (Fig. 2C).
DiscussionThis study investigated the association between diabetes and platelet reactivity using three different platelet function tests in a Korean patient population. It was found that: 1) DM patients have enhanced residual platelet activity in the overall data, but no significant difference in platelet reactivity in the PSM data when compared to non-DM patients as determined by the VN, with no significant differences observed in platelet reactivity by LTA and MEA between the DM and non-DM patients in the overall and PSM data; 2) DM patients have a higher incidence of HPR when compared to non-DM patients in the overall and PSM data determined by the VN, and there were no significant differences in HPR incidence between the two groups determined by MEA and LTA; and 3) the MEA assay might have less sensitivity and consistency than the VN assay, or the VN test could be overestimating platelet function relative to the MEA and LTA assays, especially in conditions of strong platelet inhibition.
DM is a recognized risk factor for cardiovascular events in PCI patients. Studies have shown that platelets in diabetic patients are usually more reactive [21,22]. As well as in DM patients, the reduced responsiveness is amplified by impaired metabolism of clopidogrel, resulting in ~40% reduced exposure to the active metabolite compared with non-DM patients [8]. These findings contribute to the higher rates of HPR observed in DM patients compared with non-DM patients. In line with previous evidence, the VN P2Y12 assay in the present study determined that HPR is more prevalent in patients with diabetes.
HPR during treatment with clopidogrel has been consistently found to be a strong risk factor for recurrent ischemic events after PCI [12,13]. Patients with DM undergoing PCI in the presence of HPR are exposed to an increased risk of peri-procedural MI [23]. Mangiacapra et al. [23], reported increased platelet reactivity measured by VN in diabetic compared to non-diabetic patients, after a 600 mg loading dose clopidogrel, prior to PCI, which was in line with our findings. Schuette et al. [24], used a loading dose of 300 mg, and found that it was not sufficient to adequately overcome increased platelet reactivity measured by MEA - the platelets were stimulated with ADP-PGE in diabetic compared to non-diabetic patients, however, our MEA assay protocol for the measurement of platelet function did not include the addition of PGE1, since some aggregation is preserved due to retained activity of the P2Y1 receptor (so our MEA assay method was inferior to the VN assay system). Moreover, Angiolillo et al. [7], reported increased platelet reactivity measured by LTA in diabetic compared to non-diabetic patients, both after long term aspirin/clopidogrel dual therapy and after a 300 mg loading dose prior to PCI, however, Sibbing et al. [25], used a loading dose of 600 mg, and found no significant difference in platelet reactivity measured by LTA between diabetic and non-diabetic patients, which was in line with our results. A more recent study by Angiolillo et al. [26], compared a 600 mg loading dose of clopidogrel to a 60 mg loading dose of prasugrel, and found higher anti-platelet responses in diabetic patients treated with prasugrel, in addition to a superior response profile. In the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus) study, although doubling the clopidogrel maintenance dose improved platelet inhibition in DM patients, 60% of the patients remained suboptimal responders, underscoring the need for alternative strategies [27]. Taken together, these results suggest that the use of alternative therapies such as ticagrelor or prasugrel could provide superior reductions in platelet reactivity in diabetic patients who do not respond sufficiently to clopidogrel. Further studies are warranted to test the concept of individualized antiplatelet treatment regimens for diabetic patients based on the degree of platelet reactivity.
The various platelet function test systems work on different principles and may be sensitive to different aspects of platelet activation. LTA was the first available platelet function test and represents the historical gold standard for platelet function assessment. The VN is a fast and standardized POC test, while MEA is considered a nearPOC assay based on impedance aggregometry. In our study, VN showed a higher detection sensitivity than both LTA and MEA in terms of platelet reactivity. Regarding correlation, the LTA assay shows a moderate correlation with both the VN and MEA assays, but the MEA and VN assays show a lower correlation. Zhang et al. [28], reported a moderate correlation between LTA and VN before or after PCI, but the correlation between MEA and VN measurements showed low values, similar to our findings. Specifically, in our results, the LTA and VN assay shows the highest correlation; this result is in agreement with previous findings reported by Zhang et al. [29]. The underlying reason could be due to the recording of light transmission as the basic principle of LTA and VN. These discordant values could also be partially explained by the presence of VN cartridges of ADP plus PGE1 as suppressors of the additional contribution of ADP-induced aggregation via the P2Y1 receptor. The VN assay is specific for monitoring ADP P2Y12 receptor inhibition and features agglutination on fibrinogen-coated beads, whereas the LTA and MEA system measure platelet aggregation in which the response to all ADP receptors is assessed [30]. Therefore, Park Y et al. [31], used MEA with added agonist concentrations of ADP at 6.4 mM and PGE1 9.4 nM, while our study specifically added only ADP at 6.4 mM. This may partly explain the inconsistent data concerning the results of the MEA assay. Also, our study did not use the same anticoagulant (MEA: hirudin anticoagulant; LTA and VN: citrate anticoagulant) for platelet function measurements. Further investigation is needed to clarify these issues.
We acknowledge several limitations to our findings. First, we cannot specify the duration of DM which is an important factor in estimating the severity of diabetes. Second, we did not perform a separate analysis for insulin-dependent patients. Third, VN was a standardized measurement, but LTA and MEA were measured using two different machines, so some variation may have been present. Finally, the platelet function test was assessed at only a single time point and platelet reactivity can change over time and may be linked to the status of glycemic control.
In conclusion, diabetic patients tended to have increased residual platelet activity as determined by the VN assay. DM patients also have a higher incidence of HPR when compared to non-DM patients as determined by the VN assay. However, there were no differences in HPR detectable by the MEA and LTA assays.
Conflicts of InterestNone of the authors declare any personal or financial conflicts of interest in relation to the data presented in this study. NotesAuthors’ contributions This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government to MHK (2022R1F1A107459512) and JYH (2022R1A2C1009739). Fig. 1.Comparison of HPR incidence in the study group determined by VerifyNow, MEA, and LTA. DM, diabetes mellitus; MEA, multiple electrode aggregometry; LTA, light transmittance aggregometry; OR, odds ratio; CI, confidence interval; PSM, propensity score matching. 
Fig. 2.Comparison of 3 different platelet function tests. (A) The correlation between MEA assay and VN assay; (B) The correlation between LTA assay and VN assay; (C) The correlation between LTA assay and MEA assay. DM, diabetes mellitus; VN, VerifyNow; MEA, multiple electrode aggregometry; LTA, light transmittance aggregometry. 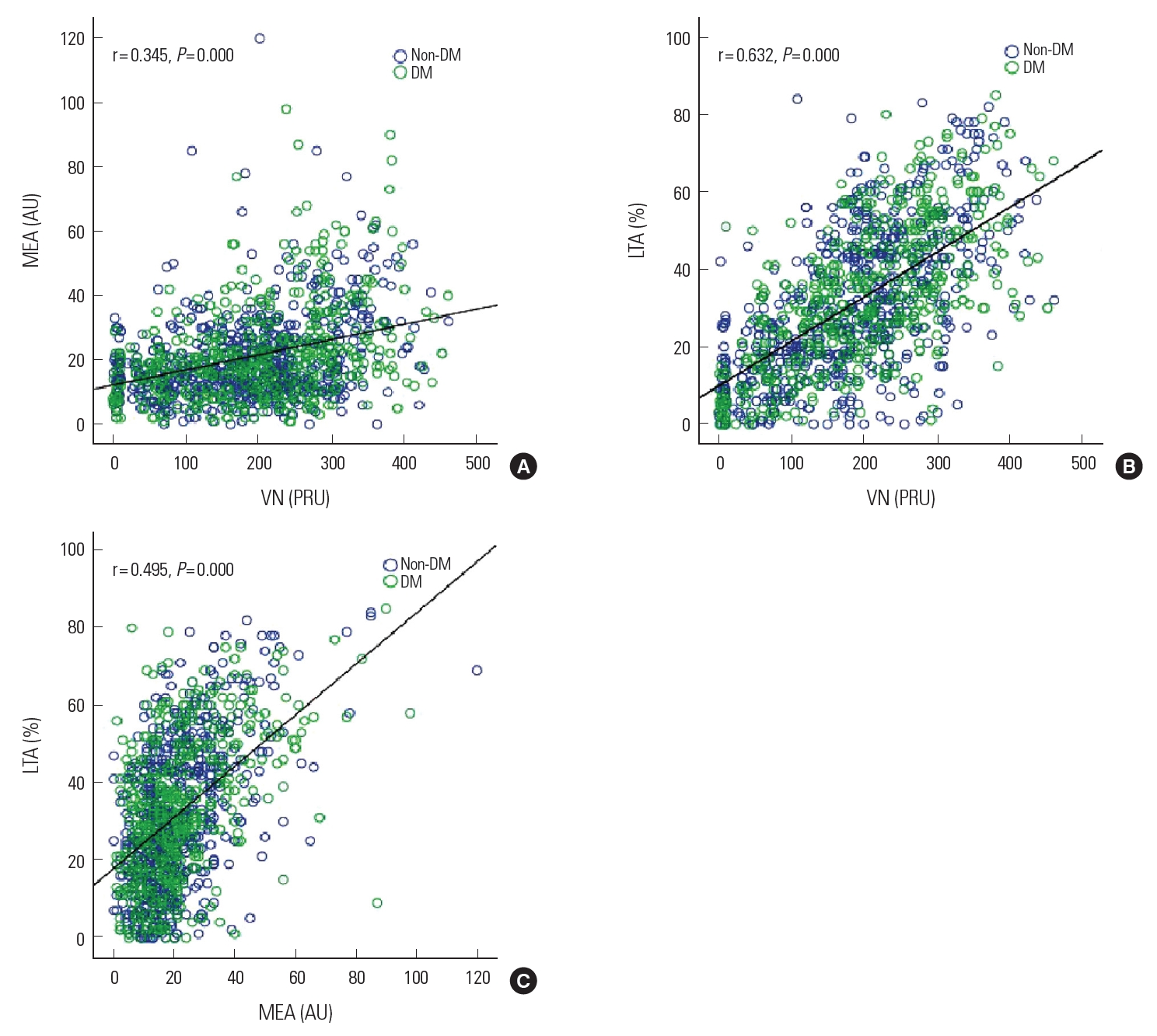
Table 1.Baseline characteristics of the study group Data are presented as number (%) or mean ± standard deviation. DM, diabetes mellitus; PSM, propensity score matching; BMI, body mass index; CKD, chronic kidney disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; AMI, acute myocardial infarction; SA, stable angina; UA, unstable angina; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; CK-MB, creatine kinase isoenzyme. Table 2.Platelet function tests and HPR percentage in the study group as determined by the different test assays Table 3.Logistic regression results from predictors of HPR determined by VN Data are presented as OR and 95% CI. OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CKD, chronic kidney disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; AMI, acute myocardial infarction; BMI, body mass index; CK-MB, creatine kinase isoenzyme. Table 4.Logistic regression results from predictors of HPR determined by MEA Data are presented as OR and 95% CI. OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CKD, chronic kidney disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; AMI, acute myocardial infarction; BMI, body mass index; CK-MB, creatine kinase isoenzyme. Table 5.Logistic regression results from predictors of HPR determined by LTA Data are presented as OR and 95% CI. OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; CKD, chronic kidney disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; AMI, acute myocardial infarction; BMI, body mass index; CK-MB, creatine kinase isoenzyme. References1. King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams SDOC, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation 2008;117(2):261-95.
2. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, et al. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Circulation 2011;123(18):2022-60.
3. Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011;32(22):2748-57.
4. Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011;123(7):798-813.
5. Rollini F, Franchi F, Muniz-Lozano A, Angiolillo DJ. Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res 2013;6(3):329-45.
6. Park Y, Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for secondary prevention in patients with diabetes mellitus and coronary artery disease. Circ J 2016;80(4):791-801.
7. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54(8):2430-5.
8. Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 2014;64(10):1005-14.
9. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350(7):664-71.
10. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation 2016;133(24):2459-502.
11. Santilli F, Simeone P, Liani R, Davi G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat 2015;120:28-39.
12. Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol 2005;46(10):1820-6.
13. Gurbel PA, Bliden KP, Navickas IA, Mahla E, Dichiara J, Suarez TA, et al. Adenosine diphosphate-induced platelet-fibrin clot strength: a new thrombelastographic indicator of long-term poststenting ischemic events. Am Heart J 2010;160(2):346-54.
14. Maiocchi S, Alwis I, Wu MCL, Yuan Y, Jackson SP. Thromboinflammatory functions of platelets in ischemia-reperfusion injury and its dysregulation in diabetes. Semin Thromb Hemost 2018;44(2):102-13.
15. Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res 2007;119(3):277-84.
16. Tóth O, Calatzis A, Penz SM, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thrombosis and Haemostasis 2006;96(6):781-8.
17. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJT, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303(8):754-62.
18. Kim HK, Tantry US, Smith SC Jr, Jeong MH, Park SJ, Kim MH, et al. The East Asian Paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost 2021;121(4):422-32.
19. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62(24):2261-73.
20. Aradi D, Storey RF, Komocsi A, Trenk D, Gulba D, Kiss RG, et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J 2014;35(4):209-15.
21. Mandal S, Sarode R, Dash S, Dash RJ. Hyperaggregation of platelets detected by whole blood platelet aggregometry in newly diagnosed noninsulin-dependent diabetes mellitus. American Journal of Clinical Pathology 1993;100(2):103-7.
22. Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 2003;26(7):2181-8.
23. Mangiacapra F, Patti G, Peace A, Gatto L, Vizzi V, Ricottini E, et al. Comparison of platelet reactivity and periprocedural outcomes in patients with versus without diabetes mellitus and treated with clopidogrel and percutaneous coronary intervention. Am J Cardiol 2010;106(5):619-23.
24. Schuette C, Steffens D, Witkowski M, Stellbaum C, Bobbert P, Schultheiss HP, et al. The effect of clopidogrel on platelet activity in patients with and without type-2 diabetes mellitus: a comparative study. Cardiovasc Diabetol 2015;14:15.
25. Sibbing D, von Beckerath O, Schömig A, Kastrati A, von Beckerath N. Diabetes mellitus and platelet function after administration of aspirin and a single 600 mg dose of clopidogrel. J Thromb Haemost 2006;4(12):2566-8.
26. Angiolillo DJ, Badimon JJ, Saucedo JF, Frelinger AL, Michelson AD, Jakubowski JA, et al. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 Trial. Eur Heart J 2011;32(7):838-46.
27. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007;115(6):708-16.
28. Zhang HZ, Kim MH, Jeong YH. Predictive values of post-clopidogrel platelet reactivity assessed by different platelet function tests on ischemic events in East Asian patients treated with PCI. Platelets 2014;25(4):292-9.
29. Zhang HZ, Kim MH, Han JY, Jeong YH. Defining predictive values using three different platelet function tests for CYP2C19 phenotype status on maintenance dual antiplatelet therapy after PCI. Platelets 2014;25(4):285-91.
|
|
||||||||||||||||||||||||||||||||||||