Successful Combined Sequential Treatment with Two Bypassing Agents in a Hemophilia A Patient with High-Responding Inhibitor Induced Refractory Bleeding
Article information
Trans Abstract
Development of a persistent presence of anti-factor VIII/IX alloantibodies (inhibitors) when treated with coagulation FVIII/FIX concentrates is a serious complication of replacement therapy occurring in people with hemophilia. Two bypassing agents, activated prothrombin complex concentrate factor (aPCC) and recombinant coagulation factor VIIa (rFVIIa), are available and have been used successfully to control the bleeding episodes of patients with high-titer inhibitors. However when used independently, some bleeding episodes are found to be refractory to these two bypassing agents. A combination serial treatment with these two bypassing agents is currently used for controlling bleeding in patients with refractory bleeding episodes, along with cautious monitoring for thromboembolism and disseminated intravascular coagulation. We report a case of hemophilia A patient with a high-responding inhibitor in whom refractory bleeding occurred after orthopedic surgery, who was successfully treated with a sequential treatment with the two bypassing agents.
Introduction
Development of a persistent presence of anti-factor VIII/IX allo-antibodies (inhibitors) when treated with coagulation FVIII/FIX concentrates is a serious complication of replacement therapy occurring in people with hemophilia. These allo-antibodies inhibit the pro-coagulant activity of FVIII/FIX and preclude the further use of homologous FVIII/FIX, which renders the control of bleeding difficult. It has been known that inhibitors develop in up to 40% of severe hemophilia A patients and in about 3% of hemophilia B patients.[1] Inhibitors are classified into high- and low-titer inhibitors. In order to differentiate between high- and low-titer inhibitors, the International Society on Thrombosis and Hemostasis (ISTH) recommended the cut-off inhibitor titer of 5 Bethesda units, because above this level stable hemostasis is difficult to achieve with high doses of FVIII/ FIX coagulation factor concentrates (CFCs).[2] Bypassing agents have been used successfully to control bleeding episodes in these patients. Two bypassing agents, activated prothrombin complex concentrates (aPCC, FEIBA™, Baxter AG, Vienna, Austria) and recombinant coagulation factor VIIa (rFVIIa, NovoSeven®, Novo Nordisk AS, Bagsvaerd, Denmark) are currently being used world-wide. However, not all bleeding episodes that occur in hemophilia patients with high-titer inhibitors are being controlled with these two bypassing agents. The effectiveness of each bypassing agent has been shown to be different and independently they controlled only 60-90% of bleeding episodes that occurred in patients with high-titer inhibitors.[3,4] Therefore, a new treatment strategy is needed for bleeding episodes that are not controlled by independent use of these two bypassing agents. The hemostatic mechanisms of these two bypassing agents in the milieu of inhibitor are different; they act at different sites of the coagulation pathway. Thrombin generation on the platelet surface, where FXa and FII play a pivotal role, was shown to be the main mechanism of action of aPCC.[5] Tissue factor (TF)-independent FX activation is known as a mechanism of action of rFVIIa. rFVIIa can directly activate FX on the activated platelet surface when a pharmacologic dose of rFVIIa is infused.[6,7] Therefore theoretically, when these two agents are used in combination, a synergistic or combined effect on hemostasis can be expected. The synergistic effect of this combination has been reported in an in vitro study[8] and several cases of successful control of bleeds with this combination treatment have been reported.[9,10] Combination treatment is currently recommended for use in cases of severe refractory bleeding in patients with hightiter inhibitors.[11] We herein report our experience with a successful combined sequential treatment of a hemophilia A patient with a highresponding inhibitor in whom refractory bleeding occurred after orthopedic surgery.
Case Report
A 32-year-old hemophilia A patient with a high-responding inhibitor was admitted for pin fixation on his left femur, fractured by a car accident three days previous. He had been continuously received follow- up at a pediatric department since he was diagnosed with severe hemophilia A at 5 years of age; a multi-domain deletion mutation in the F8 gene was the cause of the severe phenotype. He developed a high-responding inhibitor 2 years after the initiation of infusions of FVIII CFCs, Although high titer inbibitors developed bleeding episodes were relatively well-controlled with 2-3 doses of 75-100 IU/kg of aPCC or the same number of infusions of 75-90 ug/kg of rFVIIa, although the phenomenon of differential response[12] was observed during some bleeding episodes. On admission, his inhibitor titer was 1331 BU and the activated partial thromboplastin time (aPTT, range 28-45 seconds) was markedly prolonged (prothrombin time [PT], 12.0 seceonds; aPTT, 107.4 seconds). Platelet count was within the normal range and mild anemia (Hb 12.8 g/dL) was observed. One hundred U/kg of aPCC was delivered 1 hour before the surgery and thromboelastography (TEG) was obtained 30 minutes after the first infusion of aPCC. Since the results of the TEG analysis showed good hemostasis, a 100 IU/kg infusion of aPCC was planned every 12 hours for 48 hours after the surgery. The surgical procedure was successful and there was no unexpected bleeding or requirement of transfusion. However, 2 hours after surgery (6 hours after the first infusion of aPCC) oozing at the surgical site was observed and it progressively increased. The second dose of 100 IU/kg of aPCC was given to the patient 12 hours after the first dose. The TEG, which was obtained 30 minutes after the second infusion of aPCC, revealed relatively good hemostasis (Fig. 1, 1st TEG tracing). However, the oozing did not stop and the results of TEG repeated at 6 hours after the second infusion of aPCC (Fig. 1, 2nd TEG tracing) showed no hemostatic ability. Ninety μg/kg of rFVIIa was delivered immediately. The TEG obtained 30 minutes after the infusion of rFVIIa (Fig. 1, 3rd TEG tracing) showed better hemostasis compared with that after the second infusion of aPCC. The oozing finally stopped 1 hour after the infusion of rFVIIa. In contrast to the TEG tracing which was performed 6 hours after the second infusion of aPCC (Fig. 1, 2nd TEG tracing) the TEG measured six hours after the infusion of rFVIIa showed some hemostatic ability (Fig. 1, 4th TEG tracing). After this one cycle of aPCC sequential therapy, the oozing stopped completely without any recurrence. Afterwards, 100 IU/kg of aPCC was successfully given to the patient every 12 hours for 2 days and then it was tapered progressively over 12 days. He was discharged 15 days after surgery without any further complications.
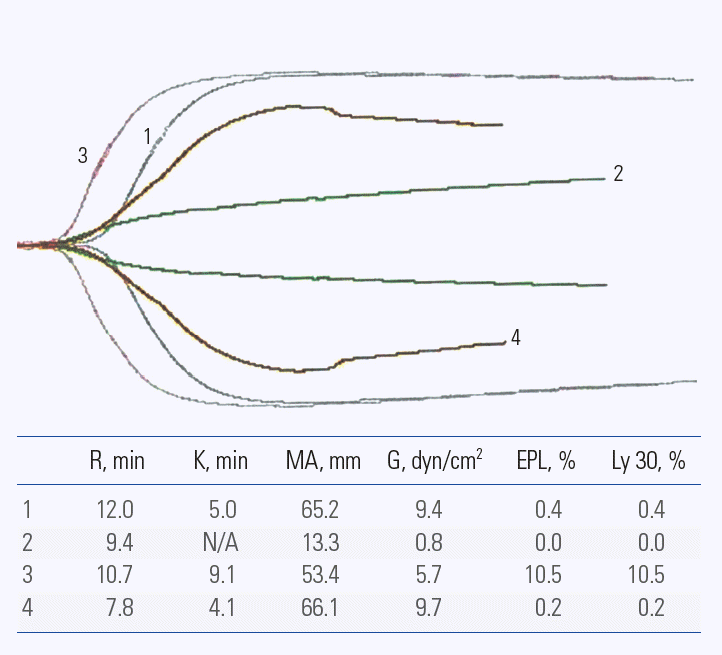
Serial follow-up thromboelastography during sequential treatment. 1. Thirty minutes after the second infusion of FEIBA-VH of 100 U/kg, 2. Six hours after the 2nd infusion of FEIBA-VH of 100 U/kg 3. Thirty minutes after the FEIBA-rFVIIa sequential therapy (6 hours after the 2nd infusion of FEIBA of 100 U/kg + 30 minutes after 90 μg/kg of rFVIIa infusion), 4. Six hours after the FEIBA-rFVIIa sequential therapy. R, reaction time; K, achievement of certain clot firmness; MA, maximum amplitude (maximum strength of the clot); G, clot strength; EPL, estimated percent lysis; Ly 30, percent lysis at 30 minutes after MA.
Discussion
Sequential treatment with bypassing agents comprises of serial infusion of two different bypassing agents to control refractory bleeding in hemophilia patients with inhibitors. The aim is to achieve a synergistic effect of the two bypassing agents on hemostasis.[8] However, the plausibility of the increased risk of thromboembolism should be considered when using the combination treatment.[9,10] These combined therapeutic strategies are generally designed to be given serially after the half-life of each of the two bypassing agents since there may be an escalated risk of thromboembolism when the two agents are given simultaneously (Table 1). In this case, the refractory bleeding occurring immediately after orthopedic surgery in a hemophilia A patient with a high-titer inhibitor was effectively controlled with only one cycle of sequential treatment without any thromboembolic complications. In this case, the combined or synergistic effect of the two bypassing agents could be observed on the TEG. Additional infusion of a bypassing agent with a different mechanism of action, rFVIIa after 6 hours of aPCC (FEIBA) infusion, demonstrated a stronger hemostasis on TEG compared with that after the aPCC infusion only, and the bleeding stopped within 1 hour of the serial combined infusion. One of the drawbacks of bypassing agents is that currently there are no reliable measurable parameters for monitoring their effect on hemostasis.[13] Recently, global coagulation tests such as thromboelastography and thrombin generation assay (TGA) were introduced to overcome this lacuna of the bypassing agents. Although there is still no global consensus regarding the use of these global assays for hemophilia, TEG and TGA are currently being used for hemophilia in many clinical settings, especially in monitoring the hemostatic effect of bypassing agents.[13] Thromboembolism is a known complication associated with the use of each of the two bypassing agents, especially in patients at risk of thrombosis, such as those with atherosclerosis.[14,15] The combined use of bypassing agents may augment the risk of thromboembolic complications associated with each of the two agents. Therefore, it is recommended not to use this therapy in patients with a risk of thromboembolism and to use it only in cases with refractory bleeding which cannot be controlled with the maximum use of one bypassing agent.[11] The concomitant use of antifibrinolytic agents should be avoided and the patients should be admitted for close surveillance for signs and symptoms of thromboembolism and disseminated intravascular coagulation while this serial combination therapy is being infused.11 Additionally, imaging studies for the detection of thrombosis should be performed and the fibrinogen and D-dimer levels should be checked daily.
