Preoperative Thrombocytosis Is an Independent Poor Prognostic Factor in Patients with Epithelial Ovarian Cancer
Article information
Trans Abstract
Purpose
Platelets may promote cancer progression through diverse mechanisms, including protection of cancer cells from immune surveillance, cancer-cell arrest in the microvasculature, and stimulation of angiogenesis. We investigated whether platelet count was related to clinical outcome in epithelial ovarian cancer (EOC).
Methods
Clinical data from 377 patients with EOC diagnosed at CHA Bundang Medical Center from January 2000 to June 2013 were retrospectively analyzed. Thrombocytosis was defined as a platelet count of more than 450,000/mm2.
Results
Stage III/IV ovarian cancer was correlated with higher median platelet counts than stage I/II disease (281,000/mm3 vs. 263,000/mm3, P= 0.002). Twenty-eight (7.5%) out of 377 patients had thrombocytosis at the time of initial diagnosis of EOC. Patients with thrombocytosis were significantly more likely to have advanced stage disease (OR= 3.288, P= 0.008) and higher preoperative levels of median cancer antigen (CA) 125 than those with normal platelet counts (221.7 vs. 28.2 ng/mL, P< 0.001). Patients with thrombocytosis had a significantly shorter median time to progression-free survival (PFS) and overall survival (OS) than those with normal platelet counts, respectively (median PFS 20.9 vs. 78.4 months, P= 0.002 and median OS 64 months vs. not reached, P= 0.001). In the multivariate analysis, the presence of thrombocytosis, advanced stage, and suboptimal cytoreduction were significantly associated with decreased OS.
Conclusion
Preoperative thrombocytosis is closely linked to poor clinical outcome in patients with EOC.
Introduction
Epithelial ovarian cancer (EOC) accounts for 3% of all cancers among women world-wide (2.2% in Korea), and ranks third among gynecologic cancers.[1,2] Despite an improvement in surgical technique and development of effective chemotherapy, the overall prognosis of patients with advanced EOC is still poor, with a 5-year survival rate of only 30-40%.[3,4] In an attempt to better estimate patient outcome, many prognostic factors have been investigated. In particular, there has been a long-standing interest in how platelets relate to cancer growth and metastasis. Thrombocytosis has been demonstrated to be an unfavorable prognostic factor in a number of malignancies, including lung, gastric, breast, and pancreatic cancers.[5-8] The relationship between malignant tumors and elevated platelet counts raises the possibility of a pathophysiologic interaction between platelets and cancer cells.[9-13] Recently, serum thrombopoietin and interleukin- 6 (IL-6) levels were shown to be elevated in patients with advanced stage EOC and thrombocytosis.[14] In addition, platelets from patients with EOC-associated thrombocytosis were shown to diminish the effect of taxane chemotherapy in vitro.[14,15] There is little known about the association between thrombocytosis and EOC in Korean women. In this study, we investigated the correlation between preoperative thrombocytosis and clinical characteristics in EOC, and examined the association with survival and other known clinicopathologic prognostic factors.
Methods
All patient data for the current study were retrieved from a database of 377 patients who were diagnosed with EOC after staging laparotomy at CHA Bundang Medical Center, CHA University between January 2000 and June 2013. This study was approved by our hospital’s Institutional Review Board. Patients with tumors of low malignant potential, histories of myeloproliferative disorders, acute inflammatory diseases, or splenectomies were excluded. For all study subjects, preoperative blood samples including platelet counts and CA-125 levels were obtained from patients with EOC within 2 weeks prior to operation. Thrombocytosis was defined as a platelet count of at least 450,000/mm3. Clinical and pathological data were collected from the medical records of enrolled patients. We collected data on age, preoperative complete blood cell count (CBC), serum CA- 125 or histological cell type and grade,surgical outcomes (optimal vs. suboptimal), date of surgery, date of progression or recurrence, date of death, date of the last follow-up visit, and patient’s status at the last visit. Optimal surgery was defined when the size of each focus of residual disease after surgery was ≤ 1 cm. OS was assessed from the date of diagnosis to the date of death from any cause, or to the date of the last visit. PFS was measured from the date of completion of chemotherapy to either the date of progression/relapse or to the date of the last visit for patients who were alive and demonstrated no disease progression. Differences in clinical and pathologic factors between patients with and without thrombocytosis were evaluated using the Pearson’s Chi-square test and Mann-Whitney U Test. Survival probabilities were estimated using the Kaplan-Meier method. The log-rank test was utilized to examine the significance of the differences in survival between groups. The Cox proportional hazards model was used to assess the significance of potential prognostic factors as predictive parameters for patient survival. Generally, for all analyses, a P<0.05 was considered to be statistically significant. All analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
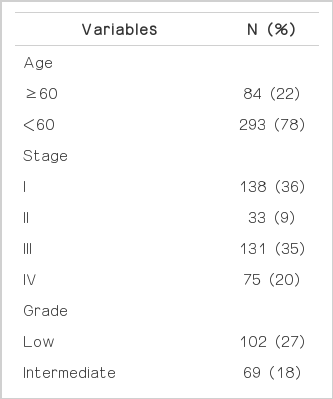
Baseline characteristics of patients and their diseases are shown in Table 1. Of the 377 patients with EOC undergoing primary surgical exploration, 28 (7.5%) had thrombocytosis. In these patients, the median platelet count was 525,000/mm3 (range, 450-786). In patients without thrombocytosis, median platelet count was 266,000/mm3 (range, 112,000-448,000/mm3). Two hundred six patients (55.5%) were diagnosed with stage III or IV disease. The most common histologic type was papillary serous (n=216, 57%). All patients underwent surgical staging by a gynecologic oncologist with the intent of optimal tumor cytoreduction. Two hundred forty-one patients (64%) had optimal tumor resection to residual disease less than 1 cm. Advanced stage disease was significantly more likely to have higher preoperative platelet counts and levels of CA-125 (P=0.002 in platelet; P<0.001 in CA-125, Fig. 1). Patients with thrombocytosis had significantly higher levels of preoperative CA-125 compared to those without thrombocytosis (P<0.001, Fig. 2). Twenty-two (76%) of the patients with thrombocytosis had advanced stage disease (odds ratio=3.288, P=0.008).
Thrombocytosis and survival
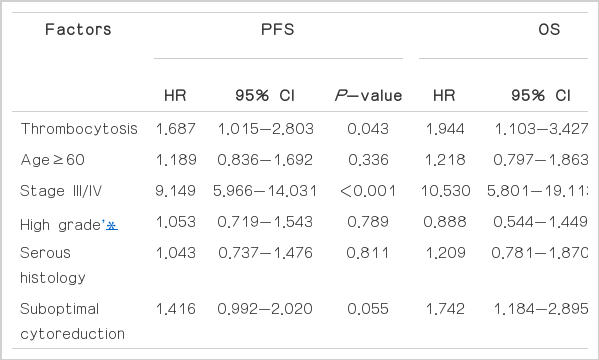
Patients with thrombocytosis had a significantly shorter median time to PFS than those with normal platelet counts (median PFS 20.9 vs. 78.4 months, P= 0.002) (Fig. 3A). The median OS among patients with thrombocytosis was 64 months, which was significantly shorter than that of patients with normal platelet counts (P=0.001) (Fig. 3B). We focused on advanced stage disease in Fig. 4. Patients with thrombocytosis had a significantly shorter PFS (median, 8.9 months) than patients without thrombocytosis (median, 24.6 months, P=0.021). We also found this relationship with OS; the median survival was 25.8 months for patients with thrombocytosis, vs. 65.2 months for patients without it (P=0.029, Fig. 4). The Cox proportional hazards model was used to evaluate the effect of thrombocytosis on survival for patients with EOC, while adjusting for known clinical and pathologic prognostic factors (Table 2). Among all variables (age, presence or absence of thrombocytosis, stage, tumor grade, histology and optimal vs. suboptimal cytoreduction), the presence of thrombocytosis (HR=1.944; 95% confidence intervals [C.I], 1.103-3.427; P=0.021), advanced stage, and suboptimal cytoreduction were significantly associated with decreased OS.
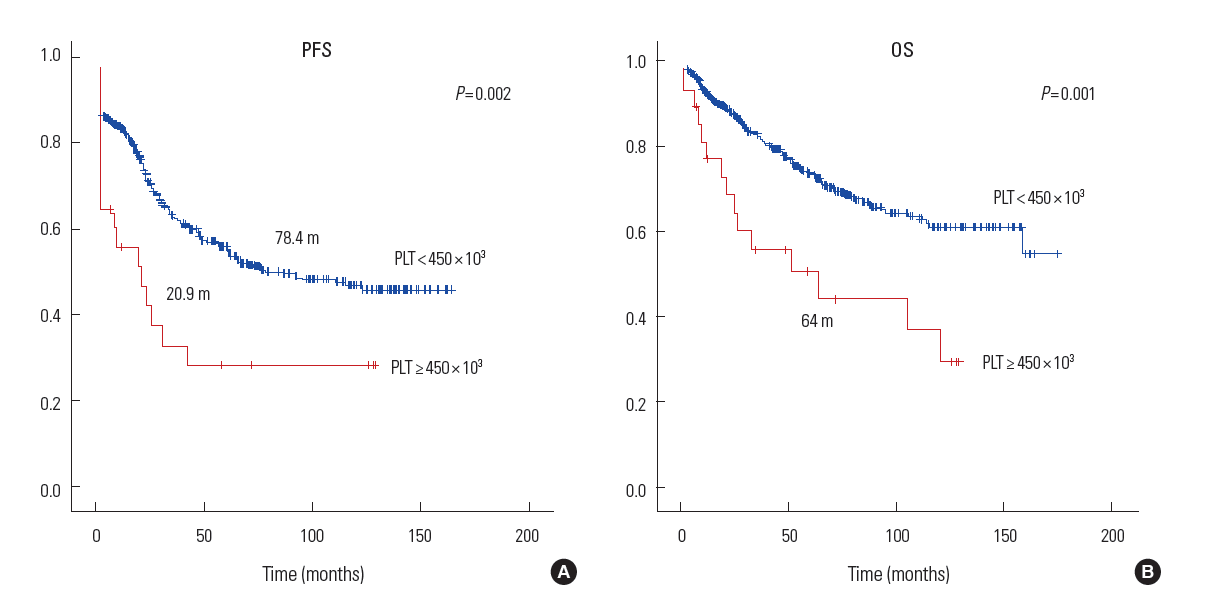
Progression-free survival (PFS) (A) and overall surval (OS) (B) of patients with epithelial ovarian cancer.

Progression-free survival (PFS) (A) and overall surval (OS) (B) of patients with advanced epithelial ovarian cancer (stage III/IV).
Discussion
The relationship between thrombocytosis and malignancy has been well demonstrated. Recently, there have been some papers on the relationship between thrombocytosis and EOC. Allensworth et al.[16] investigated 587 women with epithelial ovarian tumors, 127 of whom (22%) had early stage (stage I and II) disease. They determined that thrombocytosis was associated with advanced stage disease, existence of ascites, lower hemoglobin, and the number of packed red blood cell transfusions. In particular, their report differed from ours in that thrombocytosis was a potent predictor of worse PFS and OS only in early stage disease, not in advanced stage disease. Li et al.[17] found significantly higher rates of thrombocytosis in patients with advanced disease (stage III or IV) than in those with early disease (stage I or II). They also discovered that PFS and OS rates were significantly affected by the presence of thrombocytosis in advanced stage disease. Gungor et al.[18] carried out a similar retrospective study in a cohort of 292 patients with EOC, and they reported a higher incidence of thrombocytosis (42.5%) in women with advanced stage disease, and thrombocytosis was significantly correlated with higher levels of preoperative CA-125, more advanced disease, higher grade tumors, and shorter periods of survival. Ma et al.[19] found that the incidence of thrombocytosis was 24.7% in a cohort of 182 women diagnosed with epithelial ovarian tumors. EOC patients with thrombocytosis were found to have significantly higher grade, more advanced stage, higher level CA-125 and greater likelihood of suboptimal cytoreduction. Subjects with thrombocytosis had notably shorter PFS and OS periods. In this study, we demonstrated that thrombocytosis was associated with shortened survival and advanced stage disease, and we confirmed that thrombocytosis was significant as a poor prognostic factor in a multivariate subset analysis in Korean patients with EOC. Taken together, these data suggest that thrombocytosis is associated with factors reflective of a more aggressive tumor biology, and predict poorer survival in women with EOC. In addition, the prevalence of thrombocytosis in EOC patients in our study was low (7.5%) in comparison to other studies, ranging from 30% to 60%.[14,16-19] The low prevalence may be due to the difference in our study population and the fact that far more patients with early stage diseases (45%) were included in our study. Overall, our results are consistent with those ofthe previous reports mentioned above. In particular, our study was focused on an Asian population, specifically Korean patients, and only one other such study has previously been conducted. Platelets are highly reactive cellular effectors of hemostasis, immunity, and inflammation.[20] Also, cancer cells activate and aggregate platelets.[9-13] Platelets contain bioactive molecules and growth factors in their granules, including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin- like growth factor (IGF), transforming growth factor (TGF)-α, IL-1, IL-8, CXC motif-containing ligand 12 (CXCL12), sphingosine- 1-phosphate (S1P), and lysophosphatidic acid.[11,21] Each of these factors may actively contribute to metastatic tumor progression. For example, platelet-derived TGF-α promotes theepithelial to mesenchymal transition (EMT) program in tumor cells through the Smaand Mad-related family (Smad) and nuclear factor kappa-light-chainenhancer of activated B cell (NF-κB) signaling in these cells.[12] EMT is a program that endows epithelial cells with a mesenchymal phenotype that promotes mobility and metastasis, while protecting against apoptosis due to the lack of adhesion.[22] In addition, platelets from patients with cancer have a higher VEGF level in comparison to platelets from individuals without cancer.[23] Circulating tumor cells may use platelets as a protective shield to block their antigenic determinants from an immune system attack, or as an intermediary to help them attach to endothelial cells at the destination sites of metastases. In addition, heteroaggregates consisting of platelets and tumor cells may embolize in the microcirculation and aid in the process of extravasation of tumor cells in metastatic sites. Platelets also have roles in carcinogenesis directly related to their normal function in promotion of vascular integrity.[13,24] There are some limitations to our study. A relatively small number of patients were included in the analysis. In addition, this retrospective chart review may have unmeasured confounders such as environmental factors (smoking, alcohol, food, exercise, etc.), cause of death, herbal medication, or drugs that affect platelets.
Aggressive ovarian cancer may be linked to the presence of thrombocytosis. In ovarian cancer, elevated levels of IL-6 are associated with thrombocytosis, and this elevated platelet count leads to elevated TGF-β1, which is secreted from platelets. This secretion enhances EMT in cancer cells thus promoting metastasis, and furthermore, platelets increase the proliferation of ovarian cancer cells.[14,15] In a mouse model, IL-6 produced in ovarian cancer cells stimulated the liver to generate thrombopoietin, by which thrombopoiesis was increased via provocation of megakaryocyte progenitors in the bone marrow. Through this process, the increased platelets in turn promoted tumor growth, creating a feed-forward loop. In addition, silencing both thrombopoietin and IL-6 expression resulted in resolution of thrombocytosis, and neutralizing IL-6 made EOC more sensitive to taxane therapy.[14,15] Recent large studies have revealed that daily use of aspirin after the diagnosis of colorectal cancer decreases cancer-specific and overall mortality[25] and low molecular-weight heparin improves survival among patients with cancer.[26] Antiplatelet strategies may have the potential to serve as a valuable therapeutic approach for malignancies. In conclusion, preoperative thrombocytosis is associated with more aggressive tumor biology, and is predictive of poorer survival in Korean women with EOC. Further validation of the potential clinical application of thrombocytosis to predict clinical outcomes in EOC is necessary.
Acknowledgements
This work was supported by grants from the Korean Ministry of Science and Education, NRF-2012-007-678, 2013-RIA-1A-2060778.
Notes
There are no conflicts of interest relevant to this article to report.