Essentials of Laboratory Issues in the Patients with Emicizumab Therapy
Article information
Trans Abstract
The recent development of emicizumab (Hemlibra, previously referred to as ACE910; Hoffman-La Roche) extends treatment options for hemophilia A patients, with and without anti-factor VIII (FVIII) inhibitors, and provides an alternative to FVIII replacement therapy for patients with severe hemophilia A. The novel nature and mode of action of the molecule have implications for laboratory testing of coagulation parameters in patients receiving this treatment. Emicizumab is an engineered IgG4 bispecific antibody that binds both activated factor IX (FIXa) and its substrate factor X (FX). This interaction colocalizes components of the intrinsic tenase complex and improves the ability of FIXa to activate FX in the absence of activated FVIII (FVIIIa). Therefore, FVIII is a mimetic because it acts as a cofactor for FIXa activation of FX. In a recently published study, information regarding the influence of emicizumab on certain coagulation tests, measurement of FVIII in the presence of emicizumab, and measurement of emicizumab levels was reported. In the present review, the issues associated with laboratory testing in hemophilia A patients receiving emicizumab were summarized.
Introduction
Hemophilia is the most common bleeding disorder in South Korea, according to the 2018 Korean Prevalence Survey [1,2]. Hemophilia A accounts for approximately 70% of cases, of which 97% are in the uninhibited form; the inhibited form, which occurs as an autoimmune response to treatment, is very rare [3]. Hemophilia is classified into mild, moderate, and severe forms based on the activity of coagulation factors VIII (FVIII) and IX (FIX). The recent development of emicizumab (Hemlibra, previously referred to as ACE910; Hoffman-La Roche) extends treatment options for hemophilia A patients and provides an alternative to FVIII replacement therapy for patients with severe hemophilia A [4,5]. The novel nature and mode of action of the molecule have implications for laboratory testing of coagulation parameters in patients receiving this treatment.
Assays used to aid in the management of hemophilia A
Two tests are used in the management of patients with hemophilia A: the one-step FVIII activity test and the FVIII inhibitor test known as the Bethesda assay.
The one-step FVIII activity test serves three purposes. First, it is used to initially assess the severity of the disease, and patients are categorized as mild, moderate, or severe based on the test results. Second, when therapeutic drugs are used, properly adjusting the drug dosage is necessary, and a decrease in the activity of FVIII during drug use indicates the occurrence of FVIII inhibitors. Third, to assess the coagulation status before surgery or procedures, and if the preoperative test shows very low activity of FVIII, the use of additional therapeutic agents should be considered to reduce the risk of bleeding. The activated partial thromboplastin time (aPTT) detects fibrin clot formation by adding a phospholipid, calcium known as partial thromboplastin (PT), and clot activator to patient plasma. In the one-step clot-based FVIII activity test, patient plasma is mixed with manufactured plasma selectively deficient in FVIII to ensure clotting time is dependent on FVIII present only in the patient’s plasma. Next, the aPTT test is performed to determine the ratio of the measured clotting time to a normal measurement, and the ratio is defined as FVIII activity.
The FVIII inhibitor test is performed to detect FVIII inhibitors, which can be produced by exogenous supply of FVIII. As a control reaction, when manufactured plasma lacking FVIII is mixed 1:1 with normal plasma, the activity of FVIII is 50%. This is defined as no inhibitor present state. If an inhibitor is present in the patient’s plasma, the FVIII activity will be reduced to less than 50% when tested after mixing 1:1 with normal plasma. One Bethesda unit of inhibitor is defined as 50% inhibition of FVIII in normal plasma, resulting in 25% of FVIII activity.
Principle and benefits of emicizumab
Emicizumab is a mimetic bispecific antibody that binds to activated factor IX (FIXa) and factor X (FX) and can act as an activated FVIII (FVIIIa) by itself. In vivo, FVIII must be activated to initiate a clotting response. Although existing hemophilia A treatments act as FVIII, emicizumab can directly act as FVIIIa without the FVIII activating process [6,7].
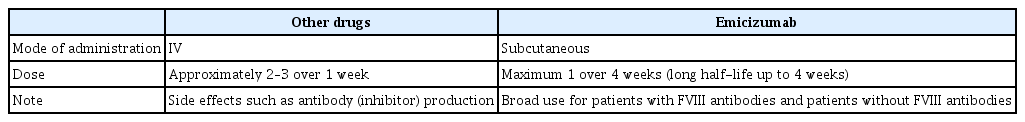
Emicizumab has several advantages over conventional agents. First, emicizumab can be administered subcutaneously, unlike conventional agents that must be administered intravenously. Second, emicizumab has a relatively long half-life compared with conventional agents, which allows it to be administered every 4 weeks without significant changes in bioavailability [8]. Last, emicizumab can be administered in the presence or absence of FVIII inhibitors without the side effects reported with existing agents, such as generation of FVIII inhibitors (Table 1).
Emicizumab does not require activation for its cofactor activity, unlike FVIII
Due to differences in the effects of FVIII and emicizumab on the coagulation process, the coagulation mechanism is limited to activation of FVIII in normal circumstances, which then acts between FIXa and FXa to initiate the coagulation process. Simultaneously, anticoagulation occurs through thrombin. Conversely, emicizumab can act as FVIIIa, avoiding the time necessary for FVIII to be activated, and anticoagulation caused by thrombin does not occur, which can be especially useful for assays reflecting intrinsic pathways.
Emicizumab interferes with certain laboratory assays
The use of emicizumab may affect the results of certain coagulation tests [9], including aPTT, one-stage aPTT-based single-factor assays such as the FVIII activity test, FVIII inhibitor tests based on clotting time such as the Bethesda assay, activated protein C resistance tests based on aPTT, and activated clotting time. In patients using emicizumab, clotting time-based tests are affected, and clinical decisions should not be made based on test results.
Coagulation tests affected by emicizumab should be replaced by those that are not affected by emicizumab, such as ELISA or latex particle-based immunoassays, chromogenic assays, and assays promoted by thrombin. Coagulation tests that are weakly affected by emicizumab are PT-derived INR and derived fibrinogen assay. In contrast, the coagulation tests that are strongly affected by emicizumab include aPTT-based assays, such as aPTT, protein C activity, protein S activity, and activated protein C resistance test. This is due to emicizumab’s mechanism of action interfering with aPTT-based tests such as the aPTT-based single-factor assay, Bethesda assay, and activated clotting time.
Emicizumab has a strong effect on aPTT and leads to overestimated results in the one-stage FVIII assay
The aPTT is one of the coagulation tests most highly affected by emicizumab, and the activity of FVIII is important in determining the rate of the aPTT. Emicizumab acts as an activated FVIII and does not require activation. As shown in Fig. 1A, normal aPTT results were observed in patients receiving emicizumab at concentrations of 2–5 μg/mL, which is significantly below the therapeutic concentration (Fig. 1A). Therefore, normal-range aPTT results for a patient receiving emicizumab therapy do not reflect the patient’s true coagulation status and may be misinterpreted.
Emicizumab does not require an activation step and the clotting mechanism proceeds more rapidly than the traditional method. When a one-step FVIII assay is performed with emicizumab-spiked plasma, high FVIII activity is reported. As shown in Fig. 1B, the emicizumab concentration with 100% FVIII activity is 10 μg/mL, which is below the therapeutic level. At therapeutic emicizumab concentrations, the one-stage FVIII assay will show results greater than 150 and cannot be used to measure emicizumab concentrations (Fig. 1B).
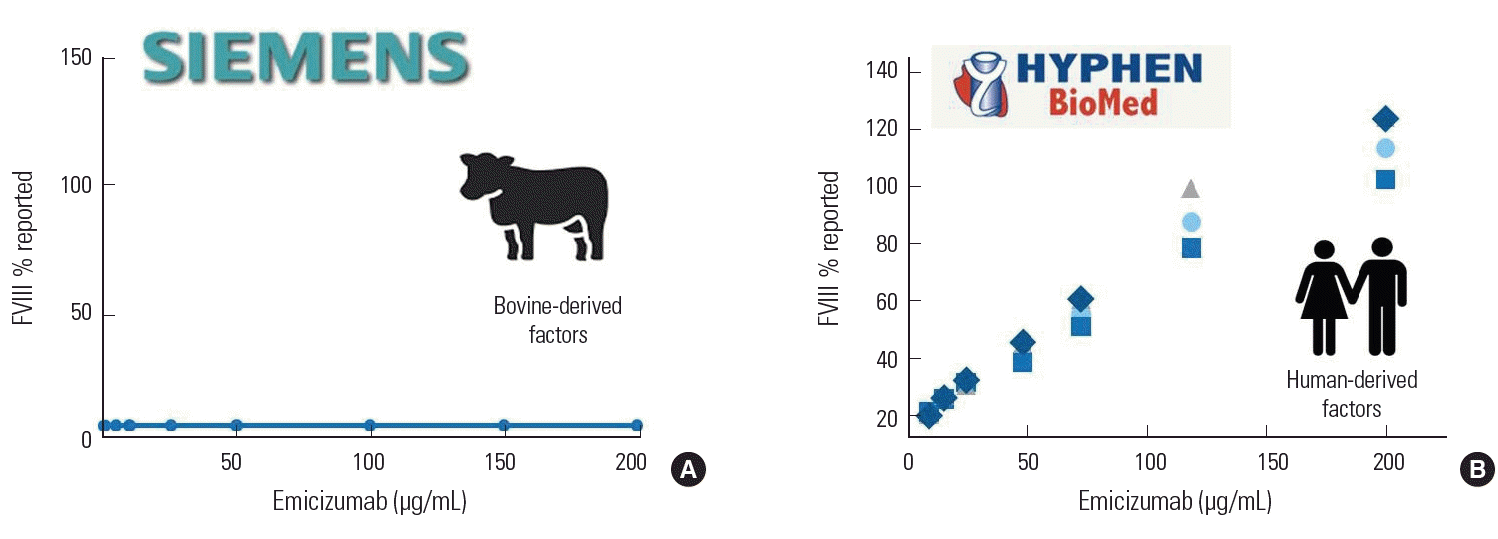
Chromogenic FVIII assays are only sensitive to emicizumab if they use human-derived components
In several studies, the chromogenic method, which measures activation in terms of chromogenicity, has been suggested instead of the clotting method [10,11]. Two types of chromogenic FVIII reagents exist depending on whether the FIXa and FXa components of the reagent are bovine- or human-derived. The bovine-derived reagent is a Siemens product and is not affected by emicizumab; therefore, it can be used to measure FVIII activity in patients receiving emicizumab. Human-derived reagents are Biomed HYPHEN products, are sensitive to emicizumab, and should be used to monitor emicizumab level after treatment (Fig. 2).
Summary of the effect of emicizumab on aPTT and FVIII activity assays
The two main coagulation tests are affected in patients receiving emicizumab. First, aPTT can be measured as normal at very low emicizumab concentrations, indicating overreactivity and an inaccurate result. Second, the clotting-based FVIII assay is normal at very low emicizumab concentrations, indicating overreactivity and an inaccurate result. Third, the bovine-based chromogenic FVIII assay is insensitive to emicizumab and can accurately measure a patient’s FVIII activity. However, this assay cannot be used to measure emicizumab activity in the body. Finally, the human-based chromogenic FVIII assay is sensitive to emicizumab and cannot accurately measure the patient’s FVIII activity. However, this assay can be used to measure emicizumab activity in the body (Table 2) [10-12].
The effect of emicizumab on FVIII inhibitor assays
Emicizumab may also affect the FVIII inhibitor test. If an FVIII inhibitor is present in a patient with severe hemophilia A, the inhibitor is currently tested using the Bethesda assay. In the presence of emicizumab, the assay reacts with the clotting reagent and results in an over-estimation of FVIII activity, which is reported as no inhibitor present because FVIII activity is not reduced below 50%. If the bovine-based chromogenic Bethesda assay is used, the assay may prevent the reagent from reacting with emicizumab due to its insensitivity. Consequently, the increase of FVIII activity can be prevented, allowing detection of the inhibitors that cause the activity of FVIII to drop below 50%. Because emicizumab reacts with human-derived FIXa and FXa but not with bovine-derived FIXa and FXa, the Bethesda assay, involving binding between human-derived coagulation factors and emicizumab, an produce false-negative results, such as no inhibitor present. However, when using the bovine proteinbased chromogenic Bethesda assay, binding to emicizumab does not occur and the presence of an inhibitor can be detected [9,13,14].
Bethesda assay versus chromogenic Bethesda assay in the presence of emicizumab
When measuring FVIII activity, chromogenic FVIII activity tests containing bovine proteins can be used to measure endogenous or infused FVIII activity. However, these tests do not detect emicizumab and cannot be used to measure emicizumab activity. When measuring FVIII inhibitors, the chromogenic Bethesda assay containing bovine protein can be used to measure FVIII inhibitors, However, in clotting-based Bethesda assays, false-negative results can occur, limiting the use of this assay to measure FVIII inhibitors. All clotting-based assays overestimate emicizumab hemostatic activity and cannot be used to monitor emicizumab activity.
Recommended coagulation testing methods for patients receiving emicizumab
The aPTT-based tests are unsuitable for the measurement of coagulation factors or inhibitors in patients being treated with emicizumab, and alternative non-aPTT based assays should be used. Local verification of PT reagent response should be considered for measurement of PT in patients treated with emicizumab.
FVIII level, if required in patients being treated with emicizumab, should be measured using an FVIII chromogenic assay that uses bovine-derived FIXa and FXa components. Examples include Coamatic FVIII assay (Chromogenix), Coatest SP (Chromogenix), and FVIII Chromogenic Assay (Siemens). Verification of kits containing both bovine-derived FX and human-derived FIXa, for example Technochrom FVIII:C (Technoclone) and Rossix FVIII (Rossix), as suitable for measuring FVIII in the presence of emicizumab is recommended prior to use.
FVIII inhibitor level in patients receiving emicizumab should be measured using chromogenic reagents containing bovine-derived FIXa and FXa components during treatment as well as prior to treatment initiation. Verification of kits containing bovine-derived FX and human-derived FIXa as suitable for measuring FVIII inhibitors in the presence of emicizumab is recommended prior to use (Table 3) [10].
Summary
When interpreting unexpected assay results in patients receiving emicizumab, it is important to understand the effect on one-stage coagulation-based FVIII activity measurement, resulting in an increase > 150%. In contrast, chromogenic FVIII activity assays using bovine protein may be insensitive to emicizumab and can be used for accurate assessment of FVIII activity. In addition, the clotting-based Bethesda assay may falsely detect low FVIII inhibitor level, and a chromogenic FVIII inhibitor assay using bovine protein can be used for accurate assessment.
When monitoring emicizumab concentration, aPTT and activated clotting time cannot be used because emicizumab shortens aPTT and activated clotting time even when used at low concentrations. In this case, measurement of FVIII activity using human protein may be appropriate for monitoring emicizumab concentration.
Acknowledgements
None.